An Acid Everywhere - Phosphoric Acid (H3PO4)
The title itself best describes the phosphoric acid - The Acid Everywhere or simply call it an acid useful for almost every industry. In this blog firstly we will discuss about need and uses of phosphoric acid and then focus on manufacturing processes and related markets.
The need and uses of Phosphoric acid -
Phosphoric acid is used as an additive and flavoring agent in both human and animal feed. It is commonly used in sodas to provide a sharp or sour flavor. In fact, almost all the acidic flavor in soda comes from phosphoric acid, as the carbonic acid contained in the bubbles has little effect on the overall pH. Phosphoric acid also helps to keep bacteria and fungi from forming in these sugary drinks.
Phosphorous is one of the most essential plant nutrients. In order to add extra phosphorous to soil, phosphoric acid is converted into phosphates that are then mixed in with other ingredients to form fertilizer. More than 80 percent of the phosphoric acid produced in the world is used in the manufacture of fertilizer. As of 2014, Asia is the world's largest producer of phosphoric acid, with around 14 million tons produced per year. North America is second in production, with annual production of approximately 10 million tons.
Manufacturing Processes of H3PO4-
Phosphoric acid is produced industrially by two general routes – the thermal process and the wet process, which includes two sub-methods. The wet process dominates in the commercial sector. The more expensive thermal process produces a purer product that is used for applications in the food industry.
Wet Process-
Wet process phosphoric acid is prepared by adding sulfuric acid to tricalcium phosphate rock, typically found in nature as apatite. The reaction is:
- Ca5(PO4)3X + 5 H2SO4 + 10 H2O → 3 H3PO4 + 5 CaSO4·2 H2O + HX
- where X may include OH, F, Cl, and Br
The initial phosphoric acid solution may contain 23–33% P2O5 (32–46% H3PO4), but can be concentrated by the evaporation of water to produce acid, which contains 75–85% H3PO4. Further evaporation of water yields above 70% (corresponding to nearly 100% H3PO4. Wet-process acid can be further purified by removing fluorine to produce animal-grade phosphoric acid, or by solvent extraction and arsenic removal to produce food-grade phosphoric acid.
Thermal process -
Very pure phosphoric acid is obtained by burning elemental phosphorus to produce phosphorus pentoxide, which is subsequently dissolved in dilute phosphoric acid. This route produces a very pure phosphoric acid, since most impurities present in the rock have been removed when extracting phosphorus from the rock in a furnace. The end result is food-grade, thermal phosphoric acid; however, for critical applications, additional processing to remove arsenic compounds may be needed.
Elemental phosphorus is produced by an electric furnace. At a high temperature, a mixture of phosphate ore, silica and carbonaceous material (coke, coal etc...) produces calcium silicate, phosphorus gas and carbon monoxide. The P and CO off-gases from this reaction are cooled under water to isolate solid phosphorus. Alternatively, the P and CO off-gases can be burned with air to produce phosphorus pentoxide and carbon dioxide.
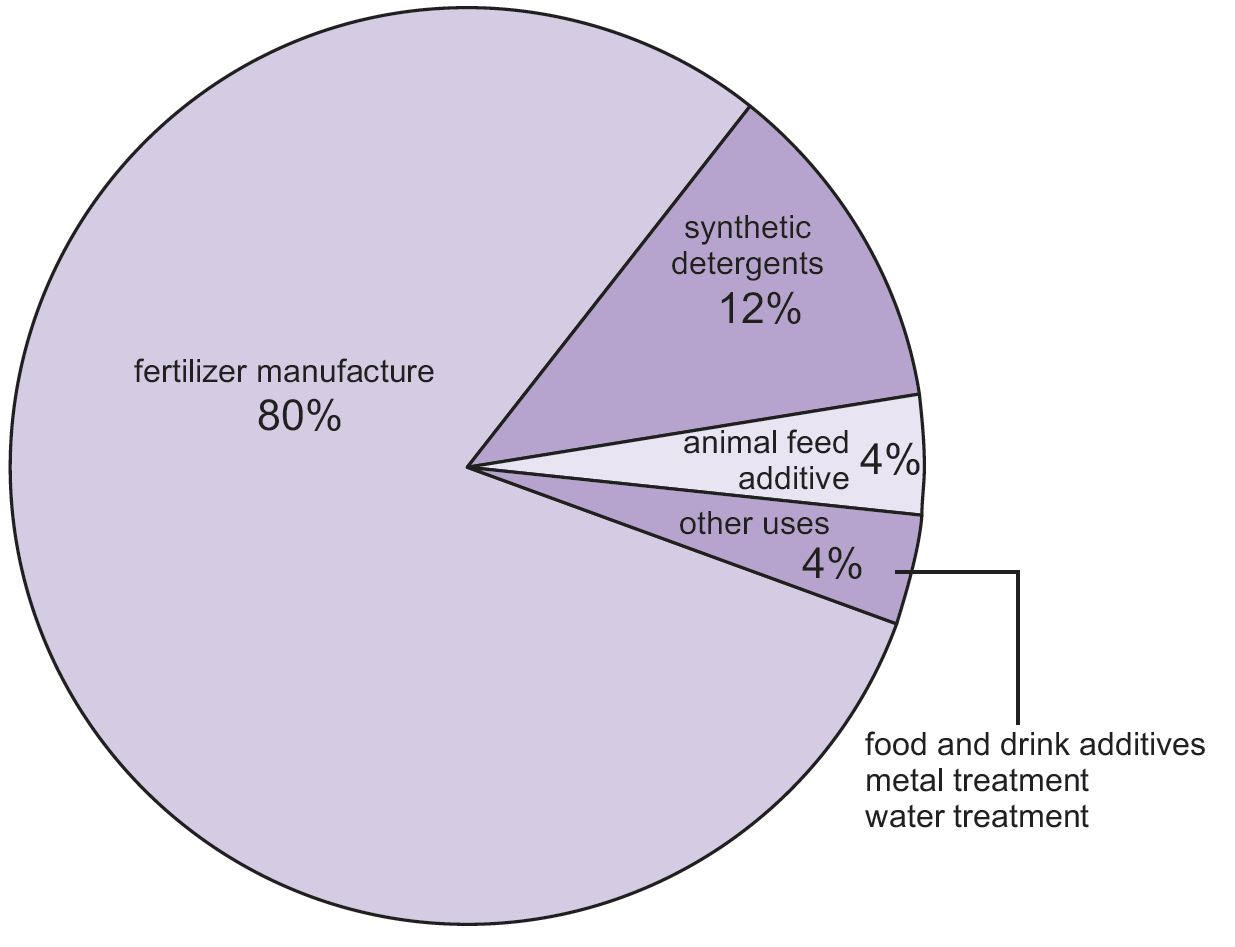
Market -
The Phos acid is used for various purposes and so is the market of it as it given in figure below -
Hope, you got all about phosphoric acid in this blog. Thank you for reading.
Keep reading, madhugb28blogs. Explore more of phosphoric acid in my coming blogs. Thank you, again.
With love phosacid.blogspot.com.
With love phosacid.blogspot.com.
Blogger- madhugb28@gmail.com

No comments:
Post a Comment